Contract Manufacturing ERP: Top Development ERP Software for Life Sciences
Digital Transformation in the Development and Contract Manufacturing Market
Adaptability is Key
CDMOs, CMOs, and CROs are faced with meeting the demands of multiple customers, products, and batch sizes. Technology and modern equipment play a large role in allowing CDMOs to remain flexible and assist current and future customers.
Contract Development and Manufacturing Organizations come to Navigator when they find their ability to grow and/or adapt is being limited by their current business processes and systems. Navigator helps accelerate operational performance through digital transformation in CDMOs so our customers can drive innovation, grow their business, become a leader in the industry, and increase market share. With the help of ERP software, manufacturing businesses can better meet the demands of their customers.
"With Navigator by their side, CBM’s ERP was up and running within 10 weeks, an impressive feat considering the complexity of the situation.” Read on. . . - Steve Gwynn,Director of Enterprise Systems at CBM
Supporting the Rapid Expansion with Enterprise Resource Planning
Strategic updates in technology, such as implementing enterprise resource planning (ERP) software, are what CDMOs must strive for to efficiently increase future capacity, deliver consistently to customers and uphold regulatory guidelines.
As pharmamanufacturing.com reports, in 2020, the U.S. Department of Health and Human Services and the U.S. Department of Defense called upon CDMOs to support the expansion of the United States’ capacity for manufacturing and distributing vaccines or therapeutics related to the COVID-19 pandemic.
To remain competitive in the growing CDMO industry, it may be time to consider the power of flexible technology in your organization by implementing an ERP system. Manufacturing ERP software can help contract manufacturers control every stage of their product lifestyles, from the initial stage of development and manufacturing to inventory and order management.

Cloud-built ERP software solutions, specific for CDMOs
“ERP systems are not known for going in quickly and effectively. On the front, it seems simple… but it’s very complex because there are so many integrated areas. If you don’t do it right, it can fall apart quickly. We needed to be able to do this right the first time. With Navigator’s expertise, it worked out well.” Read on. . .
- Steve Gwynn, Director of Enterprise Systems at CBM
-45.jpg?width=450&height=300&name=7_27_21_DiscoveryLabs(Lab)-45.jpg)
Client SUCCESS STORIES with contract Manufacturing Software
Scott Harris
Vice President & Site Director
TriRx Pharmaceutical Services

Client SUCCESS STORIES with contract Manufacturing Software
Steve Gwynn
Director of Enterprise Systems
Center for Breakthrough Medicines

We’ve Helped Other Life Science Organizations Meet Their Manufacturing Business Goals
As biotech and pharmaceutical materials become increasingly complex. . .
Life Science organizations are facing a growing number of hurdles in developing and manufacturing substances, building blocks, and finished formulas. Increased innovation and production timelines are needed to bring solutions to patients faster while managing expense levels, or even reducing costs, which is top of mind for many pharmaceutical and biotechnology companies.
With the high cost of equipment, facility development, and an increasing range of equipment needed, sterile liquid dosage, liquid, and semi-solid dosage, and solid dosage forms, organizations are looking at ways to balance quality, speed, and cost of business operations.
Working with a contract development and manufacturing organization (CDMO), contract manufacturing organization (CMO), or contract research organization (CRO) can help life science companies bring new products or formulas to market faster while lowering the investment risk inherent in building out additional internal infrastructure.
A CDMO is a contract development and manufacturing organization, they not only handle the outsourced manufacturing of biological, nutraceutical, and pharmaceutical materials, but they are also involved in some if not all of the innovation and development work prior to the manufacturing process. From the initial product details to inventory management, a contract manufacturer can help any life science business with their manufacturing needs.
As the need for contract manufacturers continues to expand, it is essential for the contract companies to have management software that can meet the growing demand. Navigator offers high-quality ERP management systems that can help manufacturing businesses meet their needs. Businesses can successfully control their whole business workflow with our ERP management system.
To learn more about our ERP management systems, you can click request demo button for a free demo or contact us for additional information about the software.
ERP System Resources
SAP ByDesign an ERP Solution for Growth-Focused Life Science Companies
The demands of life sciences companies change as quickly as the science.
9 Reasons Why SAP Business ByDesign is Designed for Life Science Companies
SAP Business ByDesign is a great fit for a Life Sciences company for a number of reasons.
See our Industry-specific Cloud ERP Solutions in Action
Register for a solution overview demo now.
What's the Difference Between Contract Manufacturers?
Contract Manufacturing and Development Organizations (CDMOs), Contract Manufacturing Organizations (CMOs), and Contract Research Organizations (CROs) are at the center of today’s life sciences global supply chain disruption; including a dramatic increase in inventory demand, rapidly changing expectations from global drug manufacturing partners, and increasing competition from start-ups looking to capture market share back from big corporations.
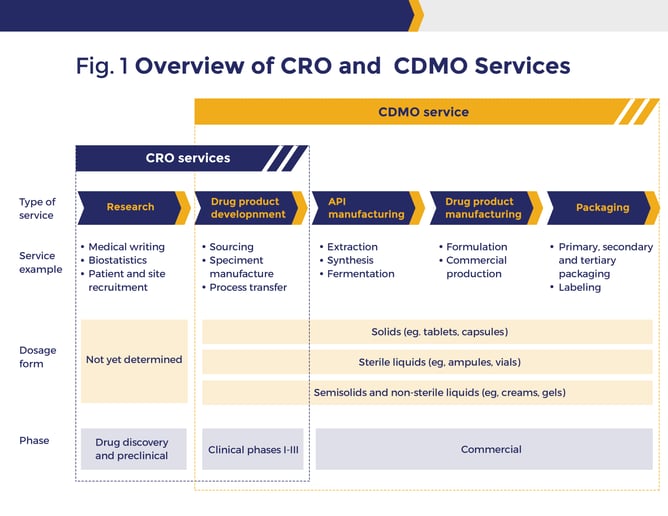
What is a CDMO?
A CDMO is a contract development and manufacturing organization, meaning they not only handle the manufacturing needs of their clients but are also part of the innovation and development process that leads to manufacturing high-quality inventory. A contract manufacturing business can manage the business from the development and raw material stage to the shop floor and distribution. These contract manufacturers can include development, production and analysis, and material sourcing, allowing their customers to limit the need to build and staff dedicated innovation and inventory manufacturing facilities.
Services often offered by CDMO companies include formulation, analytical services, blending, coating, converting, packaging, serialization, and inventory shipment. Many CDMOs based on their business focus, can start with a ready-to-go formula, or at the concept level, provide pre-formulation and formulation development services at volumes that will support both clinical trials and commercial production management.
When life science companies find the right CDMO, they gain flexibility, collaboration, and innovation services to lower process manufacturing costs and increase speed to market for new inventory. The right CDMO provides expertise and equipment at a lower overall investment that the customer does not have access to in-house. As cell and gene, pharmaceutical, and biotech components and materials become more complex and the need for innovation and production accelerates, CDMOs can provide a scalable solution delivering development and improving life science companies’ bottom lines.
Both the CMO and CDMO should be operating under current good manufacturing practices (cGMP), which is necessary for compliance with regulatory guidelines.
What is a CRO?
Contract research organizations (CRO) are hired by pharmaceutical, biotechnology, and medical device manufacturers to control clinical trials after the drug is developed internally or by the CDMO and is ready for project testing. CROs plan, coordinate, execute and supervise all processes involved in developing and running a clinical trial, including selecting a site (when applicable), recruiting participants, monitoring the trial, managing data, and more. A CRO manages feedback and requirements from multiple constituents, including manufacturers, trial sponsors, ethical committees, foundations, researchers, legal departments, trial participants and regulatory agencies. Some CDMOs are extending their service lines to offer these types of research services for your business.
What is a CMO?
A CMO is a contract manufacturing organization. CMOs are contracted by pharmaceutical, nutraceutical, and biotechnology companies to manufacture materials. The manufacturing contractor may take many forms including solution, emulsion and nano-suspension, aseptic filling, pre-filled syringe or vials, and tablets or capsules.
Pharmaceutical companies are continuing the trend towards working with a CMO partner for the production of their materials because equipment and facilities for mass production of some chemicals and for smaller batches can be very cost-prohibitive. It makes more financial sense to outsource to contract manufacturers than take on the risk and burden to invest in equipment costs for a manufacturing floor. These financial risks can be especially costly in a scenario where a product fails in clinical trials and development and production are canceled.
Why Pharmaceutical Companies are expanding their work with CDMOs and CMOs
Drug and therapy development is complex. By working with CDMOs and CMOs organizations can lower operational risk, better meet deadlines and more quickly scale up to meet production and inventory demand, save time, and lower investment for the business.
- Reduce infrastructure and manufacturing costs
- reducing or eliminating the need to invest in additional production and manufacturing space
- eliminate costs associated with purchasing specialized equipment
- Access additional production and management expertise
- researchers with specialized skills—and a wide breadth of experience in management
reduce client payroll costs.
- researchers with specialized skills—and a wide breadth of experience in management
- Meet production deadlines
- Flexibility to scale with increasing demand
- Add product variation without the added expenses of labor, facility space, etc.